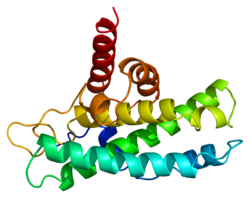
RB1
Retinoblastomski protein (pRb, Rb, RB ili RB1) je tumor-supresorski protein čija nefunkcionalnost uzrokuje nekoliko glavnih kancera.[5] Jedna od funkcija pRb je sprečavanje prekomjerne proliferacije ćelija, inhibiranjem progresije ćelijskog ciklusa sve dok ćelija nije spremna za diobu. Kad je ćelija spremna za podjelu, pRb se fosforilira, deaktivirajući je, i dopušta se ćelijskom ciklusu da napreduje. Također je regruter nekoliko enzima za remodeliranje hromatina, kao što su metilaze i acetilaze.[6]
pRb pripada porodici džepnih proteina, čiji članovi imaju džep za funkcionalno vezivanje drugih proteina.[7][8] Ako se onkogeni proteini, poput onih koje proizvode ćelije inficirane visokorizičnim tipovima ljudskog papiloma virusa, veže i inaktivira pRb, to može dovesti do raka. Gen RB je možda bio odgovoran za evoluciju višećelijskih oblika u nekoliko loza života, uključujući životinje.[9]
Ime i genetika
urediKod ljudi, protein je kodiran genom RB1 koji se nalazi na hromozomu 13, tačnije, na sekvenci 13q14.1-q14.2. Ako oba alela ovog gena mutiraju u ranom životu, protein se inaktivira i rezultira razvojem retinoblastomakog raka, pa otuda i naziv pRb (p=protein). Ćelije mrežnjače se ne odmazuju i ne zamjenjuju, te su podvrgnute visokom nivou mutagenog UV-zračenja, pa se zato većina izlučivanja pRb-a javlja u njenom tkivu (ali je također dokumentirano kod određenih karcinoma kože kod pacijenata iz New Yorka i Novog Zrelanda gdje je količina UV zračenja znatno veća).
Uočena su dva oblika retinoblastoma: bilateralni, porodični i jednostrani, sporadični oblik. Pacijenti ovih bolesti imali su šest puta veću vjerojatnoću da će kasnije u životu razviti druge tipove raka.[10] Ovo je naglasilo činjenicu da se mutirani pRb mogao naslijediti i dati podršku za hipotezu o dva pogotka. Ovo navodi na zaključak da je samo jedan aktivni alel genski supresor tumora neophodan za njegovu funkciju (mutirani gen je recesivan), pa se prije pojave fenotipa raka dogosila takva mutacija. U porodičnom obliku, mutirani alel se nasljeđuje zajedno s normalnim. U ovom slučaju, ako bi ćelija održala samo jednu mutaciju u drugom "RB" genu, svi pRb u toj ćeliji bili bi nedjelotvorni u inhibiranju progresije ćelijskog ciklusa, dopuštajući ćelijama da se nekontrolirano dijele i na kraju postanu kancerogene. Nadalje, kako je jedan alel već mutiran u svim ostalim somatskim ćelijama, buduća učestalost karcinoma kod ovih osoba javlja se prema linearnoj kinetici.[11] Aktivni alel ne mora se podvrgnuti mutaciji per se, jer se u takvim tumorima često primjećuje gubitak heterozigotnosti (LOH) .
Međutim, u sporadičnom obliku, oba alela bi morala održati mutaciju prije nego što ćelija postane kancerogena. Ovo objašnjava zašto oboljeli od sporadičnog retinoblastoma nisu u povećanom riziku od raka kasnije u životu, jer su oba alela funkcionalna u svim drugim ćelijama. Buduća učestalost karcinoma u sporadičnim slučajevima pRb-a promatrana je polinomnom, kinetikom, ne baš kvadratnom, kako se očekivalo jer prva mutacija mora nastati normalnim mehanizmima, a zatim je LOH može duplicirati tumorskim progenitorima.
RB1 ortolozi [12] identificirani su i kod većine sisara, za koje su dostupni potpuni podaci o genomu.
RB/E2F-proteini porodice potiskuju transkripciju.[13]
Struktura označava funkciju
uredipRb je multifunkcionalni protein s mnogim mjestima vezanja i fosforilacija. Iako se njegova zajednička funkcija smatra vezanjem i potiskivanjem ciljeva E2F, pRb je vjerovatno višenamjenski protein jer se veže na najmanje 100 drugih proteina.[14]
pRb ima tri glavne strukturne komponente: karboksi-kraj, "džepnu" podjedinicu i amino-kraj. Unutar svakog domena postoje različita mjesta vezivanja proteina, kao i ukupno 15 mogućih mjesta fosforilacije. Općenito, fosforilacija uzrokuje zaključavanje među domenima, što mijenja konformaciju pRb i sprječava vezanje za ciljne proteine. Različita mjesta mogu se fosforilirati u različito vrijeme, što dovodi do mnogih mogućih konformacija i vjerovatno mnogih funkcija/nivoa aktivnosti.[15]
- Aminokiselinska sekvenca
Dužina polipeptidnog lanca je 928 aminokiselina, a molekulska težina 106.159 Da.[16].
| 10 | 20 | 30 | 40 | 50 | ||||
|---|---|---|---|---|---|---|---|---|
| MPPKTPRKTA | ATAAAAAAEP | PAPPPPPPPE | EDPEQDSGPE | DLPLVRLEFE | ||||
| ETEEPDFTAL | CQKLKIPDHV | RERAWLTWEK | VSSVDGVLGG | YIQKKKELWG | ||||
| ICIFIAAVDL | DEMSFTFTEL | QKNIEISVHK | FFNLLKEIDT | STKVDNAMSR | ||||
| LLKKYDVLFA | LFSKLERTCE | LIYLTQPSSS | ISTEINSALV | LKVSWITFLL | ||||
| AKGEVLQMED | DLVISFQLML | CVLDYFIKLS | PPMLLKEPYK | TAVIPINGSP | ||||
| RTPRRGQNRS | ARIAKQLEND | TRIIEVLCKE | HECNIDEVKN | VYFKNFIPFM | ||||
| NSLGLVTSNG | LPEVENLSKR | YEEIYLKNKD | LDARLFLDHD | KTLQTDSIDS | ||||
| FETQRTPRKS | NLDEEVNVIP | PHTPVRTVMN | TIQQLMMILN | SASDQPSENL | ||||
| ISYFNNCTVN | PKESILKRVK | DIGYIFKEKF | AKAVGQGCVE | IGSQRYKLGV | ||||
| RLYYRVMESM | LKSEEERLSI | QNFSKLLNDN | IFHMSLLACA | LEVVMATYSR | ||||
| STSQNLDSGT | DLSFPWILNV | LNLKAFDFYK | VIESFIKAEG | NLTREMIKHL | ||||
| ERCEHRIMES | LAWLSDSPLF | DLIKQSKDRE | GPTDHLESAC | PLNLPLQNNH | ||||
| TAADMYLSPV | RSPKKKGSTT | RVNSTANAET | QATSAFQTQK | PLKSTSLSLF | ||||
| YKKVYRLAYL | RLNTLCERLL | SEHPELEHII | WTLFQHTLQN | EYELMRDRHL | ||||
| DQIMMCSMYG | ICKVKNIDLK | FKIIVTAYKD | LPHAVQETFK | RVLIKEEEYD | ||||
| SIIVFYNSVF | MQRLKTNILQ | YASTRPPTLS | PIPHIPRSPY | KFPSSPLRIP | ||||
| GGNIYISPLK | SPYKISEGLP | TPTKMTPRSR | ILVSIGESFG | TSEKFQKINQ | ||||
| MVCNSDRVLK | RSAEGSNPPK | PLKKLRFDIE | GSDEADGSKH | LPGESKFQQK | ||||
| LAEMTSTRTR | MQKQKMNDSM | DTSNKEEK |
- Simboli
Supresija ćelijskog ciklusa
uredipRb ograničava sposobnost ćelije da replicira DNK, sprečavanjem njenog napredovanja iz G1 u S (faza sinteze) .[17] pRb se veže i inhibira vezivajućeg partnera za dimerizaciju proteina (E2F-DP), koji su faktori transkripcije iz porodice E2F koji uvode ćeliju u S-fazu.[18][19][20][21][22][23] Održavajući E2F-DP inaktiviranim, RB1 održava ćeliju u G1 fazi, sprječavajući napredovanje kroz ćelijski ciklus i djelujući kao supresor rasta.[8] Kompleks pRb-E2F/DP također privlači protein zvani histon-deacetilaza (HDAC) u hromatin, smanjujući transkripciju faktora koji promoviraju S fazu, dodatno suzbijajući sintezu DNK.
Aktiviranje i deaktiviranje
urediKad dođe vrijeme za ćeliju da uđe u S fazu, kompleksi ciklin-ovisna kinaza (CDK) i ciklini fosforiliraju pRb, dopuštajući E2F-DP da se disocira od pRb i postane aktivan. Kad je E2F slobodan, aktivira faktore poput ciklina (npr. ciklin E i ciklin A), koji tjeraju ćeliju kroz ćelijski ciklus aktiviranjem kinaza ovisnih o ciklinu, i molekuli koja se naziva proliferirajući ćelijski jedarni antigen ili PCNA , koji ubrzava replikaciju DNK i nje popravak, pomažući pri vezivanju polimeraze za DNK.[24][25]
Deaktivacija
urediOd 1990-ih godina poznato je da je pRb inaktiviran fosforilacijom. Do tada je prevladavao model da ga je ciklin D-Cdk 4/6 progresivno fosforilirao iz nefosforiliranog u njegovo hiperfosforilirano stanje (14+ fosforilacija). Međutim, nedavno je pokazano da pRb postoji samo u tri stanja: nefosforiliran, monofosforiliran i hiperfosforiliran. Svaki ima jedinstvenu ćelijsku funkciju.[26]
Prije razvoja 2D IEF, samo se hiperfosforilirani pRb razlikovao od svih ostalih oblika, tj. nefosforilirani pRb nalikovao je monofosforiliranom pRb na imunoblotovima. Pošto je pRb bio ili u aktivnom „hipofosforiliranom“ stanju ili u neaktivnom „hiperfosforilisanom“ stanju. Međutim, s 2D IEF-om, sada je poznato da je pRb nefosforiliran u G0 i monofosforiliran u ranim G1 ćelijama, prije hiperfosforilacije, nakon restrikcijske tačke u kasnoj G1 fazi.
Aktivacija
urediTokom prijelaza M-u-G1, pRb se zatim progresivno defosforilira, pomoću PP1, vraćajući se u svoje hipofosforilirano stanje koje potiskuje rast.[8][27]
Proteini porodice pRb su komponente DREAM kompleksa, sastavljene od DP, E2F4/5, sličnih RB (p130/p107) i MuvB (Lin9: Lin37: Lin52: RbAbP4: Lin54). Kompleks DREAM sastavljen je u Go/G1 i održava mirovanje, sastavljanjem na promotorima > 800 gena ćelijskog ciklusa i posredovanjem transkripcijske represije. Sklapanje DREAM-a zahtijeva DYRK1A (Ser/Thr kinaza) zavisnu fosforilaciju jedarne komponente MuvB, Lin52 na serinu28. Ovaj mehanizam je ključan za regrutiranje p130/p107 u MuvB jedru, a time i sklopa DREAM.
Interakcije
urediPoznato je da pRb stupa u interakciju s više od 300 proteina, od kojih su neki navedeni u nastavku:
- Gen Abl[28][29]
- Androgenski receptor[30][31]
- Apoptoza-antagonizirajući transcripcijski faktor[32][33]
- ARID4A[34]
- Receptor aril-hidrougljika[35]
- BRCA1[36][37][38]
- BRF1[39][40]
- C-jun[41]
- C-Raf[42][43]
- CDK9[44]
- CUTL1[45]
- Ciklin A1[46]
- Ciklin D1[47][48]
- Ciklin T2[44]
- DNMT1[49]
- E2F1[18][50][51][52][53][54][55]
- E2F2,[56]
- E4F1[53]
- EID1[57][58]
- ENC1[59]
- FRK[60]
- HBP1[61]
- HDAC1[34][62][63][64][65][66][67]
- HDAC3[34][68]
- Histon-deacetilaza 2[34]
- INS[69]
- JARID1A[70][71]
- LIN9[72]
- MCM7[73]
- MORF4L1[51][74]
- MRFAP1,[51][74]
- MyoD[75][76]
- NCOA6[77]
- PA2G4[78]
- PPARG[68]
- PIK3R3[79]
- PAI2[80]
- Alfa 1 DNK-usmjerena polimeraza[81]
- PRDM2[82]
- PRKRA[83]
- Prohibitin[43][84]
- Protein promijelocitne leukemije[85]
- RBBP4[50][86]
- RBBP7[38][86]
- RBBP8[62][87]
- RBBP9[88]
- SNAPC1[89]
- SKP2[90][91]
- SNAPC3[89]
- SNW1[92]
- SUV39H1[93][94]
- TAF1[47][95][96][97]
- THOC1[98]
- TRAP1[99]
- TRIP11[100]
- UBTF[101] i
- USP4.[102]
Također pogledajte
urediReference
uredi- ^ a b c GRCh38: Ensembl release 89: ENSG00000139687 - Ensembl, maj 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000022105 - Ensembl, maj 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Murphree AL, Benedict WF (mart 1984). "Retinoblastoma: clues to human oncogenesis". Science. 223 (4640): 1028–33. Bibcode:1984Sci...223.1028L. doi:10.1126/science.6320372. PMID 6320372.
- ^ Shao Z, Robbins PD (januar 1995). "Differential regulation of E2F and Sp1-mediated transcription by G1 cyclins". Oncogene. 10 (2): 221–8. PMID 7838522.
- ^ Korenjak M, Brehm A (oktobar 2005). "E2F-Rb complexes regulating transcription of genes important for differentiation and development". Current Opinion in Genetics & Development. 15 (5): 520–7. doi:10.1016/j.gde.2005.07.001. PMID 16081278.
- ^ a b c Münger K, Howley PM (novembar 2002). "Human papillomavirus immortalization and transformation functions". Virus Research. 89 (2): 213–28. doi:10.1016/S0168-1702(02)00190-9. PMID 12445661.
- ^ Gallego J (maj 2016). "Multicellular Life Was Caused By The Same Gene That Suppresses Cancer". Kansas State University.
- ^ Kleinerman RA, Tucker MA, Tarone RE, Abramson DH, Seddon JM, Stovall M, et al. (april 2005). "Risk of new cancers after radiotherapy in long-term survivors of retinoblastoma: an extended follow-up". Journal of Clinical Oncology. 23 (10): 2272–9. doi:10.1200/JCO.2005.05.054. PMID 15800318.
- ^ Knudson AG (april 1971). "Mutation and cancer: statistical study of retinoblastoma". Proceedings of the National Academy of Sciences of the United States of America. 68 (4): 820–3. Bibcode:1971PNAS...68..820K. doi:10.1073/pnas.68.4.820. PMC 389051. PMID 5279523.
- ^ "OrthoMaM phylogenetic marker: RB1 coding sequence". Arhivirano s originala, 24. 9. 2015. Pristupljeno 4. 8. 2021.
- ^ Frolov MV, Dyson NJ (maj 2004). "Molecular mechanisms of E2F-dependent activation and pRB-mediated repression". Journal of Cell Science. 117 (Pt 11): 2173–81. doi:10.1242/jcs.01227. PMID 15126619.
- ^ Morris EJ, Dyson NJ (2001). "Retinoblastoma protein partners". Advances in Cancer Research. 82: 1–54. doi:10.1016/S0065-230X(01)82001-7. ISBN 9780120066827. PMID 11447760.
- ^ Dick FA, Rubin SM (maj 2013). "Molecular mechanisms underlying pRB protein function". Nature Reviews. Molecular Cell Biology. 14 (5): 297–306. doi:10.1038/nrm3567. PMC 4754300. PMID 23594950.
- ^ "UniProt, P06400". Pristupljeno 4. 8. 2021.
- ^ Goodrich DW, Wang NP, Qian YW, Lee EY, Lee WH (oktobar 1991). "The retinoblastoma gene product regulates progression through the G1 phase of the cell cycle". Cell. 67 (2): 293–302. doi:10.1016/0092-8674(91)90181-w. PMID 1655277. S2CID 12990398.
- ^ a b Wu CL, Zukerberg LR, Ngwu C, Harlow E, Lees JA (maj 1995). "In vivo association of E2F and DP family proteins". Molecular and Cellular Biology. 15 (5): 2536–46. doi:10.1128/mcb.15.5.2536. PMC 230484. PMID 7739537.
- ^ Funk JO, Waga S, Harry JB, Espling E, Stillman B, Galloway DA (august 1997). "Inhibition of CDK activity and PCNA-dependent DNA replication by p21 is blocked by interaction with the HPV-16 E7 oncoprotein". Genes & Development. 11 (16): 2090–100. doi:10.1016/0168-9525(97)90029-9. PMC 316456. PMID 9284048.
- ^ De Veylder L, Joubès J, Inzé D (decembar 2003). "Plant cell cycle transitions". Current Opinion in Plant Biology. 6 (6): 536–43. doi:10.1016/j.pbi.2003.09.001. PMID 14611951.
- ^ de Jager SM, Maughan S, Dewitte W, Scofield S, Murray JA (juni 2005). "The developmental context of cell-cycle control in plants". Seminars in Cell & Developmental Biology. 16 (3): 385–96. doi:10.1016/j.semcdb.2005.02.004. PMID 15840447.
- ^ Greenblatt RJ (2005). "Human papillomaviruses: Diseases, diagnosis, and a possible vaccine". Clinical Microbiology Newsletter. 27 (18): 139–45. doi:10.1016/j.clinmicnews.2005.09.001.
- ^ Sinal SH, Woods CR (oktobar 2005). "Human papillomavirus infections of the genital and respiratory tracts in young children". Seminars in Pediatric Infectious Diseases. 16 (4): 306–16. doi:10.1053/j.spid.2005.06.010. PMID 16210110.
- ^ Das SK, Hashimoto T, Shimizu K, Yoshida T, Sakai T, Sowa Y, et al. (novembar 2005). "Fucoxanthin induces cell cycle arrest at G0/G1 phase in human colon carcinoma cells through up-regulation of p21WAF1/Cip1". Biochimica et Biophysica Acta (BBA) - General Subjects. 1726 (3): 328–35. doi:10.1016/j.bbagen.2005.09.007. PMID 16236452.
- ^ Bartkova J, Grøn B, Dabelsteen E, Bartek J (februar 2003). "Cell-cycle regulatory proteins in human wound healing". Archives of Oral Biology. 48 (2): 125–32. doi:10.1016/S0003-9969(02)00202-9. PMID 12642231.
- ^ Narasimha AM, Kaulich M, Shapiro GS, Choi YJ, Sicinski P, Dowdy SF (juni 2014). "Cyclin D activates the Rb tumor suppressor by mono-phosphorylation". eLife. 3. doi:10.7554/eLife.02872. PMC 4076869. PMID 24876129.
- ^ Vietri M, Bianchi M, Ludlow JW, Mittnacht S, Villa-Moruzzi E (februar 2006). "Direct interaction between the catalytic subunit of Protein Phosphatase 1 and pRb". Cancer Cell International. 6: 3. doi:10.1186/1475-2867-6-3. PMC 1382259. PMID 16466572.
- ^ Miyamura T, Nishimura J, Yufu Y, Nawata H (februar 1997). "Interaction of BCR-ABL with the retinoblastoma protein in Philadelphia chromosome-positive cell lines". International Journal of Hematology. 65 (2): 115–21. doi:10.1016/S0925-5710(96)00539-7. PMID 9071815.
- ^ Welch PJ, Wang JY (novembar 1993). "A C-terminal protein-binding domain in the retinoblastoma protein regulates nuclear c-Abl tyrosine kinase in the cell cycle". Cell. 75 (4): 779–90. doi:10.1016/0092-8674(93)90497-E. PMID 8242749.
- ^ Lu J, Danielsen M (novembar 1998). "Differential regulation of androgen and glucocorticoid receptors by retinoblastoma protein". The Journal of Biological Chemistry. 273 (47): 31528–33. doi:10.1074/jbc.273.47.31528. PMID 9813067.
- ^ Yeh S, Miyamoto H, Nishimura K, Kang H, Ludlow J, Hsiao P, et al. (juli 1998). "Retinoblastoma, a tumor suppressor, is a coactivator for the androgen receptor in human prostate cancer DU145 cells". Biochemical and Biophysical Research Communications. 248 (2): 361–7. doi:10.1006/bbrc.1998.8974. PMID 9675141.
- ^ Bruno T, De Angelis R, De Nicola F, Barbato C, Di Padova M, Corbi N, et al. (novembar 2002). "Che-1 affects cell growth by interfering with the recruitment of HDAC1 by Rb". Cancer Cell. 2 (5): 387–99. doi:10.1016/S1535-6108(02)00182-4. PMID 12450794.
- ^ Fanciulli M, Bruno T, Di Padova M, De Angelis R, Iezzi S, Iacobini C, et al. (maj 2000). "Identification of a novel partner of RNA polymerase II subunit 11, Che-1, which interacts with and affects the growth suppression function of Rb". FASEB Journal. 14 (7): 904–12. doi:10.1096/fasebj.14.7.904. PMID 10783144. S2CID 43175069.
- ^ a b c d Lai A, Lee JM, Yang WM, DeCaprio JA, Kaelin WG, Seto E, Branton PE (oktobar 1999). "RBP1 recruits both histone deacetylase-dependent and -independent repression activities to retinoblastoma family proteins". Molecular and Cellular Biology. 19 (10): 6632–41. doi:10.1128/mcb.19.10.6632. PMC 84642. PMID 10490602.
- ^ Ge NL, Elferink CJ (august 1998). "A direct interaction between the aryl hydrocarbon receptor and retinoblastoma protein. Linking dioxin signaling to the cell cycle". The Journal of Biological Chemistry. 273 (35): 22708–13. doi:10.1074/jbc.273.35.22708. PMID 9712901.
- ^ Aprelikova ON, Fang BS, Meissner EG, Cotter S, Campbell M, Kuthiala A, et al. (oktobar 1999). "BRCA1-associated growth arrest is RB-dependent". Proceedings of the National Academy of Sciences of the United States of America. 96 (21): 11866–71. Bibcode:1999PNAS...9611866A. doi:10.1073/pnas.96.21.11866. PMC 18378. PMID 10518542.
- ^ Fan S, Yuan R, Ma YX, Xiong J, Meng Q, Erdos M, et al. (august 2001). "Disruption of BRCA1 LXCXE motif alters BRCA1 functional activity and regulation of RB family but not RB protein binding". Oncogene. 20 (35): 4827–41. doi:10.1038/sj.onc.1204666. PMID 11521194.
- ^ a b Yarden RI, Brody LC (april 1999). "BRCA1 interacts with components of the histone deacetylase complex". Proceedings of the National Academy of Sciences of the United States of America. 96 (9): 4983–8. Bibcode:1999PNAS...96.4983Y. doi:10.1073/pnas.96.9.4983. PMC 21803. PMID 10220405.
- ^ Johnston IM, Allison SJ, Morton JP, Schramm L, Scott PH, White RJ (juni 2002). "CK2 forms a stable complex with TFIIIB and activates RNA polymerase III transcription in human cells". Molecular and Cellular Biology. 22 (11): 3757–68. doi:10.1128/MCB.22.11.3757-3768.2002. PMC 133823. PMID 11997511.
- ^ Sutcliffe JE, Cairns CA, McLees A, Allison SJ, Tosh K, White RJ (juni 1999). "RNA polymerase III transcription factor IIIB is a target for repression by pocket proteins p107 and p130". Molecular and Cellular Biology. 19 (6): 4255–61. doi:10.1128/mcb.19.6.4255. PMC 104385. PMID 10330166.
- ^ Nishitani J, Nishinaka T, Cheng CH, Rong W, Yokoyama KK, Chiu R (februar 1999). "Recruitment of the retinoblastoma protein to c-Jun enhances transcription activity mediated through the AP-1 binding site". The Journal of Biological Chemistry. 274 (9): 5454–61. doi:10.1074/jbc.274.9.5454. PMID 10026157.
- ^ Wang S, Ghosh RN, Chellappan SP (decembar 1998). "Raf-1 physically interacts with Rb and regulates its function: a link between mitogenic signaling and cell cycle regulation". Molecular and Cellular Biology. 18 (12): 7487–98. doi:10.1128/mcb.18.12.7487. PMC 109329. PMID 9819434.
- ^ a b Wang S, Nath N, Fusaro G, Chellappan S (novembar 1999). "Rb and prohibitin target distinct regions of E2F1 for repression and respond to different upstream signals". Molecular and Cellular Biology. 19 (11): 7447–60. doi:10.1128/mcb.19.11.7447. PMC 84738. PMID 10523633.
- ^ a b Simone C, Bagella L, Bellan C, Giordano A (juni 2002). "Physical interaction between pRb and cdk9/cyclinT2 complex". Oncogene. 21 (26): 4158–65. doi:10.1038/sj.onc.1205511. PMID 12037672.
- ^ Gupta S, Luong MX, Bleuming SA, Miele A, Luong M, Young D, et al. (septembar 2003). "Tumor suppressor pRB functions as a co-repressor of the CCAAT displacement protein (CDP/cut) to regulate cell cycle controlled histone H4 transcription". Journal of Cellular Physiology. 196 (3): 541–56. doi:10.1002/jcp.10335. PMID 12891711. S2CID 2287673.
- ^ Yang R, Müller C, Huynh V, Fung YK, Yee AS, Koeffler HP (mart 1999). "Functions of cyclin A1 in the cell cycle and its interactions with transcription factor E2F-1 and the Rb family of proteins". Molecular and Cellular Biology. 19 (3): 2400–7. doi:10.1128/mcb.19.3.2400. PMC 84032. PMID 10022926.
- ^ a b Siegert JL, Rushton JJ, Sellers WR, Kaelin WG, Robbins PD (novembar 2000). "Cyclin D1 suppresses retinoblastoma protein-mediated inhibition of TAFII250 kinase activity". Oncogene. 19 (50): 5703–11. doi:10.1038/sj.onc.1203966. PMID 11126356.
- ^ Dowdy SF, Hinds PW, Louie K, Reed SI, Arnold A, Weinberg RA (maj 1993). "Physical interaction of the retinoblastoma protein with human D cyclins". Cell. 73 (3): 499–511. doi:10.1016/0092-8674(93)90137-F. PMID 8490963. S2CID 24708871.
- ^ Robertson KD, Ait-Si-Ali S, Yokochi T, Wade PA, Jones PL, Wolffe AP (juli 2000). "DNMT1 forms a complex with Rb, E2F1 and HDAC1 and represses transcription from E2F-responsive promoters". Nature Genetics. 25 (3): 338–42. doi:10.1038/77124. PMID 10888886. S2CID 10983932.
- ^ a b Nicolas E, Ait-Si-Ali S, Trouche D (august 2001). "The histone deacetylase HDAC3 targets RbAp48 to the retinoblastoma protein". Nucleic Acids Research. 29 (15): 3131–6. doi:10.1093/nar/29.15.3131. PMC 55834. PMID 11470869.
- ^ a b c Pardo PS, Leung JK, Lucchesi JC, Pereira-Smith OM (decembar 2002). "MRG15, a novel chromodomain protein, is present in two distinct multiprotein complexes involved in transcriptional activation". The Journal of Biological Chemistry. 277 (52): 50860–6. doi:10.1074/jbc.M203839200. PMID 12397079.
- ^ Choubey D, Li SJ, Datta B, Gutterman JU, Lengyel P (oktobar 1996). "Inhibition of E2F-mediated transcription by p202". The EMBO Journal. 15 (20): 5668–78. doi:10.1002/j.1460-2075.1996.tb00951.x. PMC 452311. PMID 8896460.
- ^ a b Fajas L, Paul C, Zugasti O, Le Cam L, Polanowska J, Fabbrizio E, et al. (juli 2000). "pRB binds to and modulates the transrepressing activity of the E1A-regulated transcription factor p120E4F". Proceedings of the National Academy of Sciences of the United States of America. 97 (14): 7738–43. Bibcode:2000PNAS...97.7738F. doi:10.1073/pnas.130198397. PMC 16614. PMID 10869426.
- ^ Dyson N, Dembski M, Fattaey A, Ngwu C, Ewen M, Helin K (decembar 1993). "Analysis of p107-associated proteins: p107 associates with a form of E2F that differs from pRB-associated E2F-1". Journal of Virology. 67 (12): 7641–7. doi:10.1128/JVI.67.12.7641-7647.1993. PMC 238233. PMID 8230483.
- ^ Taniura H, Taniguchi N, Hara M, Yoshikawa K (januar 1998). "Necdin, a postmitotic neuron-specific growth suppressor, interacts with viral transforming proteins and cellular transcription factor E2F1". The Journal of Biological Chemistry. 273 (2): 720–8. doi:10.1074/jbc.273.2.720. PMID 9422723.
- ^ Lee C, Chang JH, Lee HS, Cho Y (decembar 2002). "Structural basis for the recognition of the E2F transactivation domain by the retinoblastoma tumor suppressor". Genes & Development. 16 (24): 3199–212. doi:10.1101/gad.1046102. PMC 187509. PMID 12502741.
- ^ Miyake S, Sellers WR, Safran M, Li X, Zhao W, Grossman SR, et al. (decembar 2000). "Cells degrade a novel inhibitor of differentiation with E1A-like properties upon exiting the cell cycle". Molecular and Cellular Biology. 20 (23): 8889–902. doi:10.1128/MCB.20.23.8889-8902.2000. PMC 86544. PMID 11073989.
- ^ MacLellan WR, Xiao G, Abdellatif M, Schneider MD (decembar 2000). "A novel Rb- and p300-binding protein inhibits transactivation by MyoD". Molecular and Cellular Biology. 20 (23): 8903–15. doi:10.1128/MCB.20.23.8903-8915.2000. PMC 86545. PMID 11073990.
- ^ Kim TA, Lim J, Ota S, Raja S, Rogers R, Rivnay B, et al. (maj 1998). "NRP/B, a novel nuclear matrix protein, associates with p110(RB) and is involved in neuronal differentiation". The Journal of Cell Biology. 141 (3): 553–66. doi:10.1083/jcb.141.3.553. PMC 2132755. PMID 9566959.
- ^ Craven RJ, Cance WG, Liu ET (septembar 1995). "The nuclear tyrosine kinase Rak associates with the retinoblastoma protein pRb". Cancer Research. 55 (18): 3969–72. PMID 7664264.
- ^ Lavender P, Vandel L, Bannister AJ, Kouzarides T (juni 1997). "The HMG-box transcription factor HBP1 is targeted by the pocket proteins and E1A". Oncogene. 14 (22): 2721–8. doi:10.1038/sj.onc.1201243. PMID 9178770.
- ^ a b Dick FA, Sailhamer E, Dyson NJ (maj 2000). "Mutagenesis of the pRB pocket reveals that cell cycle arrest functions are separable from binding to viral oncoproteins". Molecular and Cellular Biology. 20 (10): 3715–27. doi:10.1128/MCB.20.10.3715-3727.2000. PMC 85672. PMID 10779361.
- ^ Fuks F, Burgers WA, Brehm A, Hughes-Davies L, Kouzarides T (januar 2000). "DNA methyltransferase Dnmt1 associates with histone deacetylase activity". Nature Genetics. 24 (1): 88–91. doi:10.1038/71750. PMID 10615135. S2CID 20428600.
- ^ Puri PL, Iezzi S, Stiegler P, Chen TT, Schiltz RL, Muscat GE, et al. (oktobar 2001). "Class I histone deacetylases sequentially interact with MyoD and pRb during skeletal myogenesis". Molecular Cell. 8 (4): 885–97. doi:10.1016/S1097-2765(01)00373-2. PMID 11684023.
- ^ Wang S, Fusaro G, Padmanabhan J, Chellappan SP (decembar 2002). "Prohibitin co-localizes with Rb in the nucleus and recruits N-CoR and HDAC1 for transcriptional repression". Oncogene. 21 (55): 8388–96. doi:10.1038/sj.onc.1205944. PMID 12466959.
- ^ Luo RX, Postigo AA, Dean DC (februar 1998). "Rb interacts with histone deacetylase to repress transcription". Cell. 92 (4): 463–73. doi:10.1016/S0092-8674(00)80940-X. PMID 9491888. S2CID 18857544.
- ^ Ferreira R, Magnaghi-Jaulin L, Robin P, Harel-Bellan A, Trouche D (septembar 1998). "The three members of the pocket proteins family share the ability to repress E2F activity through recruitment of a histone deacetylase". Proceedings of the National Academy of Sciences of the United States of America. 95 (18): 10493–8. Bibcode:1998PNAS...9510493F. doi:10.1073/pnas.95.18.10493. PMC 27922. PMID 9724731.
- ^ a b Fajas L, Egler V, Reiter R, Hansen J, Kristiansen K, Debril MB, et al. (decembar 2002). "The retinoblastoma-histone deacetylase 3 complex inhibits PPARgamma and adipocyte differentiation". Developmental Cell. 3 (6): 903–10. doi:10.1016/S1534-5807(02)00360-X. PMID 12479814.
- ^ Radulescu RT, Bellitti MR, Ruvo M, Cassani G, Fassina G (januar 1995). "Binding of the LXCXE insulin motif to a hexapeptide derived from retinoblastoma protein". Biochemical and Biophysical Research Communications. 206 (1): 97–102. doi:10.1006/bbrc.1995.1014. PMID 7818556.
- ^ Chan SW, Hong W (juli 2001). "Retinoblastoma-binding protein 2 (Rbp2) potentiates nuclear hormone receptor-mediated transcription". The Journal of Biological Chemistry. 276 (30): 28402–12. doi:10.1074/jbc.M100313200. PMID 11358960.
- ^ Kim YW, Otterson GA, Kratzke RA, Coxon AB, Kaye FJ (novembar 1994). "Differential specificity for binding of retinoblastoma binding protein 2 to RB, p107, and TATA-binding protein". Molecular and Cellular Biology. 14 (11): 7256–64. doi:10.1128/mcb.14.11.7256. PMC 359260. PMID 7935440.
- ^ Gagrica S, Hauser S, Kolfschoten I, Osterloh L, Agami R, Gaubatz S (novembar 2004). "Inhibition of oncogenic transformation by mammalian Lin-9, a pRB-associated protein". The EMBO Journal. 23 (23): 4627–38. doi:10.1038/sj.emboj.7600470. PMC 533054. PMID 15538385.
- ^ Sterner JM, Dew-Knight S, Musahl C, Kornbluth S, Horowitz JM (maj 1998). "Negative regulation of DNA replication by the retinoblastoma protein is mediated by its association with MCM7". Molecular and Cellular Biology. 18 (5): 2748–57. doi:10.1128/mcb.18.5.2748. PMC 110654. PMID 9566894.
- ^ a b Leung JK, Berube N, Venable S, Ahmed S, Timchenko N, Pereira-Smith OM (oktobar 2001). "MRG15 activates the B-myb promoter through formation of a nuclear complex with the retinoblastoma protein and the novel protein PAM14". The Journal of Biological Chemistry. 276 (42): 39171–8. doi:10.1074/jbc.M103435200. PMID 11500496.
- ^ Mal A, Sturniolo M, Schiltz RL, Ghosh MK, Harter ML (april 2001). "A role for histone deacetylase HDAC1 in modulating the transcriptional activity of MyoD: inhibition of the myogenic program". The EMBO Journal. 20 (7): 1739–53. doi:10.1093/emboj/20.7.1739. PMC 145490. PMID 11285237.
- ^ Gu W, Schneider JW, Condorelli G, Kaushal S, Mahdavi V, Nadal-Ginard B (februar 1993). "Interaction of myogenic factors and the retinoblastoma protein mediates muscle cell commitment and differentiation". Cell. 72 (3): 309–24. doi:10.1016/0092-8674(93)90110-C. PMID 8381715. S2CID 21581966.
- ^ Goo YH, Na SY, Zhang H, Xu J, Hong S, Cheong J, et al. (februar 2004). "Interactions between activating signal cointegrator-2 and the tumor suppressor retinoblastoma in androgen receptor transactivation". The Journal of Biological Chemistry. 279 (8): 7131–5. doi:10.1074/jbc.M312563200. PMID 14645241.
- ^ Xia X, Cheng A, Lessor T, Zhang Y, Hamburger AW (maj 2001). "Ebp1, an ErbB-3 binding protein, interacts with Rb and affects Rb transcriptional regulation". Journal of Cellular Physiology. 187 (2): 209–17. doi:10.1002/jcp.1075. PMID 11268000. S2CID 42721280.
- ^ Xia X, Cheng A, Akinmade D, Hamburger AW (mart 2003). "The N-terminal 24 amino acids of the p55 gamma regulatory subunit of phosphoinositide 3-kinase binds Rb and induces cell cycle arrest". Molecular and Cellular Biology. 23 (5): 1717–25. doi:10.1128/MCB.23.5.1717-1725.2003. PMC 151709. PMID 12588990.
- ^ Darnell GA, Antalis TM, Johnstone RW, Stringer BW, Ogbourne SM, Harrich D, Suhrbier A (septembar 2003). "Inhibition of retinoblastoma protein degradation by interaction with the serpin plasminogen activator inhibitor 2 via a novel consensus motif". Molecular and Cellular Biology. 23 (18): 6520–32. doi:10.1128/MCB.23.18.6520-6532.2003. PMC 193706. PMID 12944478.
- ^ Takemura M, Kitagawa T, Izuta S, Wasa J, Takai A, Akiyama T, Yoshida S (novembar 1997). "Phosphorylated retinoblastoma protein stimulates DNA polymerase alpha". Oncogene. 15 (20): 2483–92. doi:10.1038/sj.onc.1201431. PMID 9395244.
- ^ Buyse IM, Shao G, Huang S (maj 1995). "The retinoblastoma protein binds to RIZ, a zinc-finger protein that shares an epitope with the adenovirus E1A protein". Proceedings of the National Academy of Sciences of the United States of America. 92 (10): 4467–71. Bibcode:1995PNAS...92.4467B. doi:10.1073/pnas.92.10.4467. PMC 41965. PMID 7538672.
- ^ Simons A, Melamed-Bessudo C, Wolkowicz R, Sperling J, Sperling R, Eisenbach L, Rotter V (januar 1997). "PACT: cloning and characterization of a cellular p53 binding protein that interacts with Rb". Oncogene. 14 (2): 145–55. doi:10.1038/sj.onc.1200825. PMID 9010216.
- ^ Wang S, Nath N, Adlam M, Chellappan S (juni 1999). "Prohibitin, a potential tumor suppressor, interacts with RB and regulates E2F function". Oncogene. 18 (23): 3501–10. doi:10.1038/sj.onc.1202684. PMID 10376528.
- ^ Alcalay M, Tomassoni L, Colombo E, Stoldt S, Grignani F, Fagioli M, et al. (februar 1998). "The promyelocytic leukemia gene product (PML) forms stable complexes with the retinoblastoma protein". Molecular and Cellular Biology. 18 (2): 1084–93. doi:10.1128/mcb.18.2.1084. PMC 108821. PMID 9448006.
- ^ a b Qian YW, Lee EY (oktobar 1995). "Dual retinoblastoma-binding proteins with properties related to a negative regulator of ras in yeast". The Journal of Biological Chemistry. 270 (43): 25507–13. doi:10.1074/jbc.270.43.25507. PMID 7503932.
- ^ Fusco C, Reymond A, Zervos AS (august 1998). "Molecular cloning and characterization of a novel retinoblastoma-binding protein". Genomics. 51 (3): 351–8. doi:10.1006/geno.1998.5368. PMID 9721205.
- ^ Woitach JT, Zhang M, Niu CH, Thorgeirsson SS (august 1998). "A retinoblastoma-binding protein that affects cell-cycle control and confers transforming ability". Nature Genetics. 19 (4): 371–4. doi:10.1038/1258. PMID 9697699. S2CID 11374970.
- ^ a b Hirsch HA, Gu L, Henry RW (decembar 2000). "The retinoblastoma tumor suppressor protein targets distinct general transcription factors to regulate RNA polymerase III gene expression". Molecular and Cellular Biology. 20 (24): 9182–91. doi:10.1128/MCB.20.24.9182-9191.2000. PMC 102176. PMID 11094070.
- ^ Ji P, Jiang H, Rekhtman K, Bloom J, Ichetovkin M, Pagano M, Zhu L (oktobar 2004). "An Rb-Skp2-p27 pathway mediates acute cell cycle inhibition by Rb and is retained in a partial-penetrance Rb mutant". Molecular Cell. 16 (1): 47–58. doi:10.1016/j.molcel.2004.09.029. PMID 15469821.
- ^ Wang H, Bauzon F, Ji P, Xu X, Sun D, Locker J, et al. (januar 2010). "Skp2 is required for survival of aberrantly proliferating Rb1-deficient cells and for tumorigenesis in Rb1+/- mice". Nature Genetics. 42 (1): 83–8. doi:10.1038/ng.498. PMC 2990528. PMID 19966802.
- ^ Prathapam T, Kühne C, Banks L (decembar 2002). "Skip interacts with the retinoblastoma tumor suppressor and inhibits its transcriptional repression activity". Nucleic Acids Research. 30 (23): 5261–8. doi:10.1093/nar/gkf658. PMC 137971. PMID 12466551.
- ^ Nielsen SJ, Schneider R, Bauer UM, Bannister AJ, Morrison A, O'Carroll D, et al. (august 2001). "Rb targets histone H3 methylation and HP1 to promoters". Nature. 412 (6846): 561–5. Bibcode:2001Natur.412..561N. doi:10.1038/35087620. PMID 11484059. S2CID 4378296.
- ^ Vandel L, Nicolas E, Vaute O, Ferreira R, Ait-Si-Ali S, Trouche D (oktobar 2001). "Transcriptional repression by the retinoblastoma protein through the recruitment of a histone methyltransferase". Molecular and Cellular Biology. 21 (19): 6484–94. doi:10.1128/MCB.21.19.6484-6494.2001. PMC 99795. PMID 11533237.
- ^ Shao Z, Ruppert S, Robbins PD (april 1995). "The retinoblastoma-susceptibility gene product binds directly to the human TATA-binding protein-associated factor TAFII250". Proceedings of the National Academy of Sciences of the United States of America. 92 (8): 3115–9. Bibcode:1995PNAS...92.3115S. doi:10.1073/pnas.92.8.3115. PMC 42115. PMID 7724524.
- ^ Siegert JL, Robbins PD (januar 1999). "Rb inhibits the intrinsic kinase activity of TATA-binding protein-associated factor TAFII250". Molecular and Cellular Biology. 19 (1): 846–54. doi:10.1128/MCB.19.1.846. PMC 83941. PMID 9858607.
- ^ Shao Z, Siegert JL, Ruppert S, Robbins PD (juli 1997). "Rb interacts with TAF(II)250/TFIID through multiple domains". Oncogene. 15 (4): 385–92. doi:10.1038/sj.onc.1201204. PMID 9242374.
- ^ Durfee T, Mancini MA, Jones D, Elledge SJ, Lee WH (novembar 1994). "The amino-terminal region of the retinoblastoma gene product binds a novel nuclear matrix protein that co-localizes to centers for RNA processing". The Journal of Cell Biology. 127 (3): 609–22. doi:10.1083/jcb.127.3.609. PMC 2120229. PMID 7525595.
- ^ Chen CF, Chen Y, Dai K, Chen PL, Riley DJ, Lee WH (septembar 1996). "A new member of the hsp90 family of molecular chaperones interacts with the retinoblastoma protein during mitosis and after heat shock". Molecular and Cellular Biology. 16 (9): 4691–9. doi:10.1128/MCB.16.9.4691. PMC 231469. PMID 8756626.
- ^ Chang KH, Chen Y, Chen TT, Chou WH, Chen PL, Ma YY, et al. (august 1997). "A thyroid hormone receptor coactivator negatively regulated by the retinoblastoma protein". Proceedings of the National Academy of Sciences of the United States of America. 94 (17): 9040–5. Bibcode:1997PNAS...94.9040C. doi:10.1073/pnas.94.17.9040. PMC 23019. PMID 9256431.
- ^ Hannan KM, Hannan RD, Smith SD, Jefferson LS, Lun M, Rothblum LI (oktobar 2000). "Rb and p130 regulate RNA polymerase I transcription: Rb disrupts the interaction between UBF and SL-1". Oncogene. 19 (43): 4988–99. doi:10.1038/sj.onc.1203875. PMID 11042686.
- ^ Blanchette P, Gilchrist CA, Baker RT, Gray DA (septembar 2001). "Association of UNP, a ubiquitin-specific protease, with the pocket proteins pRb, p107 and p130". Oncogene. 20 (39): 5533–7. doi:10.1038/sj.onc.1204823. PMID 11571651.
Dopunska literatura
uredi- Momand J, Wu HH, Dasgupta G (januar 2000). "MDM2--master regulator of the p53 tumor suppressor protein". Gene. 242 (1–2): 15–29. doi:10.1016/S0378-1119(99)00487-4. PMID 10721693.
- Zheng L, Lee WH (2003). "Retinoblastoma tumor suppressor and genome stability". Advances in Cancer Research Volume 85. Advances in Cancer Research. 85. str. 13–50. doi:10.1016/S0065-230X(02)85002-3. ISBN 978-0-12-006685-8. PMID 12374284.
- Classon M, Harlow E (decembar 2002). "The retinoblastoma tumour suppressor in development and cancer". Nature Reviews. Cancer. 2 (12): 910–7. doi:10.1038/nrc950. PMID 12459729. S2CID 22937378.
- Lai H, Ma F, Lai S (januar 2003). "Identification of the novel role of pRB in eye cancer". Journal of Cellular Biochemistry. 88 (1): 121–7. doi:10.1002/jcb.10283. PMID 12461781. S2CID 34538683.
- Simin K, Wu H, Lu L, Pinkel D, Albertson D, Cardiff RD, Van Dyke T (februar 2004). "pRb inactivation in mammary cells reveals common mechanisms for tumor initiation and progression in divergent epithelia". PLOS Biology. 2 (2): E22. doi:10.1371/journal.pbio.0020022. PMC 340938. PMID 14966529.
- Lohmann DR, Gallie BL (august 2004). "Retinoblastoma: revisiting the model prototype of inherited cancer". American Journal of Medical Genetics. Part C, Seminars in Medical Genetics. 129C (1): 23–8. doi:10.1002/ajmg.c.30024. PMID 15264269. S2CID 41922148.
- Clemo NK, Arhel NJ, Barnes JD, Baker J, Moorghen M, Packham GK, et al. (august 2005). "The role of the retinoblastoma protein (Rb) in the nuclear localization of BAG-1: implications for colorectal tumour cell survival". Biochemical Society Transactions. 33 (Pt 4): 676–8. doi:10.1042/BST0330676. PMID 16042572.
- Rodríguez-Cruz M, del Prado M, Salcedo M (2006). "[Genomic retinoblastoma perspectives: implications of tumor supressor [sic] gene RB1]". Revista de Investigacion Clinica. 57 (4): 572–81. PMID 16315642.
- Knudsen ES, Knudsen KE (juli 2006). "Retinoblastoma tumor suppressor: where cancer meets the cell cycle". Experimental Biology and Medicine. 231 (7): 1271–81. doi:10.1177/153537020623100713. PMID 16816134. S2CID 29078799.
Vanjski linkovi
uredi- RB1 protein, human na US National Library of Medicine Medical Subject Headings (MeSH)
- Retinoblastoma genes na US National Library of Medicine Medical Subject Headings (MeSH)
- GeneReviews/NIH/NCBI/UW entry on Retinoblastoma
- Retinoblastoma Genetics
- Drosophila Retinoblastoma-family protein - The Interactive Fly
- Drosophila Retinoblastoma-family protein 2 - The Interactive Fly
- Evolutionary Homologs Retinoblastoma-family proteins - The Interactive Fly
- There is a diagram of the pRb-E2F interactions here[trajno mrtav link].